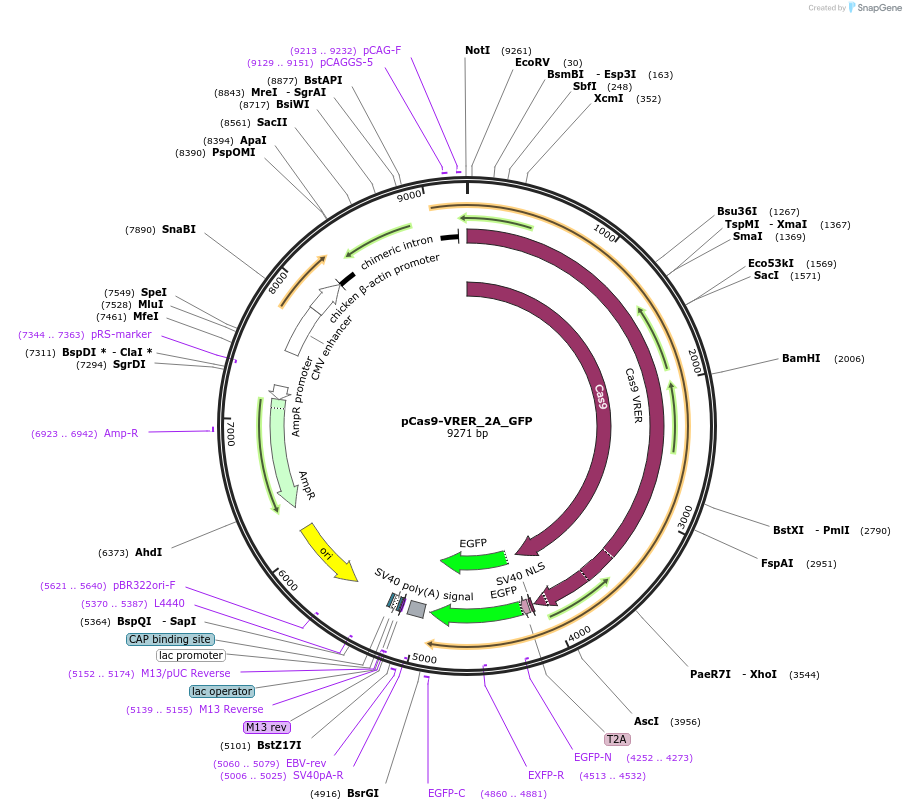
-
PurposeCas9 VRER variant that detects NGCG PAM, combined with 2A_GFP for expression control
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 75475 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $85 | |
Backbone
-
Vector backbonepCAG
- Backbone size w/o insert (bp) 4200
- Total vector size (bp) 9271
-
Vector typeMammalian Expression, CRISPR
Growth in Bacteria
-
Bacterial Resistance(s)Ampicillin, 100 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)DH5alpha
-
Copy numberUnknown
Gene/Insert
-
Gene/Insert nameCas9-VRER
-
SpeciesSynthetic; human codon-optimized
-
Insert Size (bp)4137
-
MutationD1135V, G1218R, R1335E, T1337R
- Promoter CAG
-
Tag
/ Fusion Protein
- 2A_GFP (C terminal on insert)
Cloning Information
- Cloning method Restriction Enzyme
- 5′ cloning site EcoRI (not destroyed)
- 3′ cloning site None (destroyed during cloning)
- 5′ sequencing primer ggctctagtgcctctgctaacc
- 3′ sequencing primer M13R (Common Sequencing Primers)
Resource Information
-
Supplemental Documents
-
A portion of this plasmid was derived from a plasmid made byPlasmid is modified from pCas9_GFP, originally received from Addgene (Plasmid #44719), please check MTA for original plasmid.
-
Article Citing this Plasmid
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
pCas9-VRER_2A_GFP was a gift from Marc Tessier-Lavigne (Addgene plasmid # 75475 ; http://n2t.net/addgene:75475 ; RRID:Addgene_75475) -
For your References section:
Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Paquet D, Kwart D, Chen A, Sproul A, Jacob S, Teo S, Olsen KM, Gregg A, Noggle S, Tessier-Lavigne M. Nature. 2016 May 5;533(7601):125-9. doi: 10.1038/nature17664. Epub 2016 Apr 27. 10.1038/nature17664 PubMed 27120160