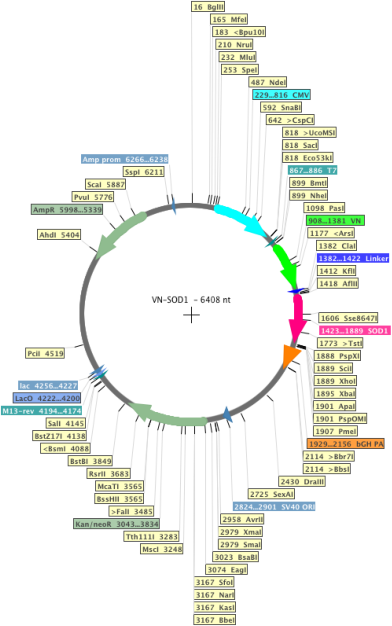
VN-SOD1
(Plasmid
#89757)
-
Purposeexpresses human SOD1 fused to the N-terminal part of Venus
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 89757 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $85 | |
Backbone
-
Vector backbonepcDNA3.1
- Backbone size w/o insert (bp) 5944
- Total vector size (bp) 6408
-
Vector typeMammalian Expression
-
Selectable markersNeomycin (select with G418)
Growth in Bacteria
-
Bacterial Resistance(s)Ampicillin, 100 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)DH5alpha
-
Copy numberHigh Copy
Gene/Insert
-
Gene/Insert nameSOD1
-
Alt nameALS, ALS1, HEL-S-44, IPOA, SOD, hSod1
-
SpeciesH. sapiens (human)
-
Insert Size (bp)464
-
GenBank IDNM_000454.4
-
Entrez GeneSOD1 (a.k.a. ALS, ALS1, HEL-S-44, IPOA, SOD, STAHP, hSod1, homodimer)
- Promoter CMV
-
Tag
/ Fusion Protein
- N-terminal part of Venus (N terminal on backbone)
Cloning Information
- Cloning method Restriction Enzyme
- 5′ cloning site AflII (not destroyed)
- 3′ cloning site XhoI (not destroyed)
- 5′ sequencing primer T7
- 3′ sequencing primer bGH (Common Sequencing Primers)
Resource Information
-
A portion of this plasmid was derived from a plasmid made byOriginal clone (VN-alpha-Synuclein) comes from Pamela J.McLean.
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
Depositor Comments
Original clone (VN-alpha Synuclein) includes already the N-terminal part of venus, and was a gift from Pamela J.McLean.
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
VN-SOD1 was a gift from Tiago Outeiro (Addgene plasmid # 89757 ; http://n2t.net/addgene:89757 ; RRID:Addgene_89757) -
For your References section:
Characterization of the activity, aggregation, and toxicity of heterodimers of WT and ALS-associated mutant Sod1. Brasil AA, de Carvalho MDC, Gerhardt E, Queiroz DD, Pereira MD, Outeiro TF, Eleutherio ECA. Proc Natl Acad Sci U S A. 2019 Dec 17;116(51):25991-26000. doi: 10.1073/pnas.1902483116. Epub 2019 Dec 3. 10.1073/pnas.1902483116 PubMed 31796595