-
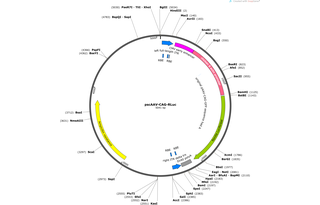
Purposerecombinant AAV vector packaging self-complementary Renilla Luciferase under the CAG promoter
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 83280 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $85 | |
Backbone
-
Vector backbonepAAV with one trs deleted ITR and CAG promoter
- Backbone size w/o insert (bp) 4105
- Total vector size (bp) 5041
-
Vector typeMammalian Expression, AAV
Growth in Bacteria
-
Bacterial Resistance(s)Ampicillin, 100 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)NEB Stable
-
Growth instructionsNeed to check for integrity of ITRs by restriction digest with AhdI, also with XmaI.
-
Copy numberHigh Copy
Gene/Insert
-
Gene/Insert nameRenilla Luciferase
-
SpeciesRenilla reniformis
-
Insert Size (bp)936
- Promoter CAG
Cloning Information
- Cloning method Restriction Enzyme
- 5′ cloning site BamH1 (not destroyed)
- 3′ cloning site EagI (not destroyed)
- 5′ sequencing primer GTTATTGTGCTGTCTCATC
- 3′ sequencing primer CCACACCTCCCCCTGAAC (Common Sequencing Primers)
Resource Information
-
Supplemental Documents
-
A portion of this plasmid was derived from a plasmid made byAAV backbone was obtained from Addgene plasmid #32396, the CAG promoter was obtained from Addgene plasmid #37825, renilla luciferase gene was obtained from Promega plasmid pRL-TK
-
Articles Citing this Plasmid
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
pscAAV-CAG-RLuc was a gift from Mark Kay (Addgene plasmid # 83280 ; http://n2t.net/addgene:83280 ; RRID:Addgene_83280) -
For your References section:
Bioengineered Viral Platform for Intramuscular Passive Vaccine Delivery to Human Skeletal Muscle. Paulk NK, Pekrun K, Charville GW, Maguire-Nguyen K, Wosczyna MN, Xu J, Zhang Y, Lisowski L, Yoo B, Vilches-Moure JG, Lee GK, Shrager JB, Rando TA, Kay MA. Mol Ther Methods Clin Dev. 2018 Jul 24;10:144-155. doi: 10.1016/j.omtm.2018.06.001. eCollection 2018 Sep 21. 10.1016/j.omtm.2018.06.001 PubMed 30101152