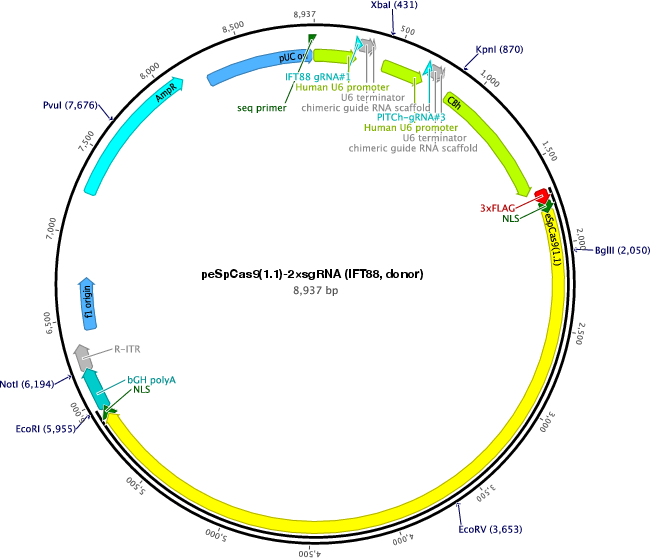
peSpCas9(1.1)-2×sgRNA (IFT88, donor)
(Plasmid
#80769)
-
PurposeExpresses eSpCas9(1.1) and two sgRNAs. The first gRNA targets human IFT88 and the second gRNA targets a pDonor-tBFP-NLS-Neo (Universal).
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 80769 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $85 | |
Backbone
-
Vector backbonepeSpCas9(1.1)-2×sgRNA (empty, donor)
-
Backbone manufacturerKazuhisa Nakayama Lab. (Addgene plasmid #80768)
- Backbone size w/o insert (bp) 8935
- Total vector size (bp) 8937
-
Modifications to backbonehuman IFT88 gRNA#1 sequence was inserted in the first sgRNA expression cassette in the plasmid.
-
Vector typeMammalian Expression, CRISPR
Growth in Bacteria
-
Bacterial Resistance(s)Ampicillin, 100 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)DH5alpha
-
Copy numberHigh Copy
Gene/Insert
-
Gene/Insert nameIFT88 gRNA#1
-
gRNA/shRNA sequenceGGGCCCCTTGACTGACTAAG
-
SpeciesH. sapiens (human)
-
GenBank IDNM_175605
-
Entrez GeneIFT88 (a.k.a. D13S1056E, DAF19, TG737, TTC10, hTg737)
Cloning Information
- Cloning method Restriction Enzyme
- 5′ cloning site BbsI (destroyed during cloning)
- 3′ cloning site BbsI (destroyed during cloning)
- 5′ sequencing primer GCTGGCCTTTTGCTCACATGTG (Common Sequencing Primers)
Resource Information
-
Supplemental Documents
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
peSpCas9(1.1)-2×sgRNA (IFT88, donor) was a gift from Kazuhisa Nakayama (Addgene plasmid # 80769 ; http://n2t.net/addgene:80769 ; RRID:Addgene_80769) -
For your References section:
Practical method for targeted disruption of cilia-related genes by using CRISPR/Cas9-mediated homology-independent knock-in system. Katoh Y, Michisaka S, Nozaki S, Funabashi T, Hirano T, Takei R, Nakayama K. Mol Biol Cell. 2017 Feb 8. pii: mbc.E17-01-0051. doi: 10.1091/mbc.E17-01-0051. 10.1091/mbc.E17-01-0051 PubMed 28179459