-
PurposeRosa26 knock-in vector for Cre-regulated cDNA expression and RNAi in transgenic mice
-
Depositing Labs
-
Publication
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 21189 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $89 | |
Backbone
-
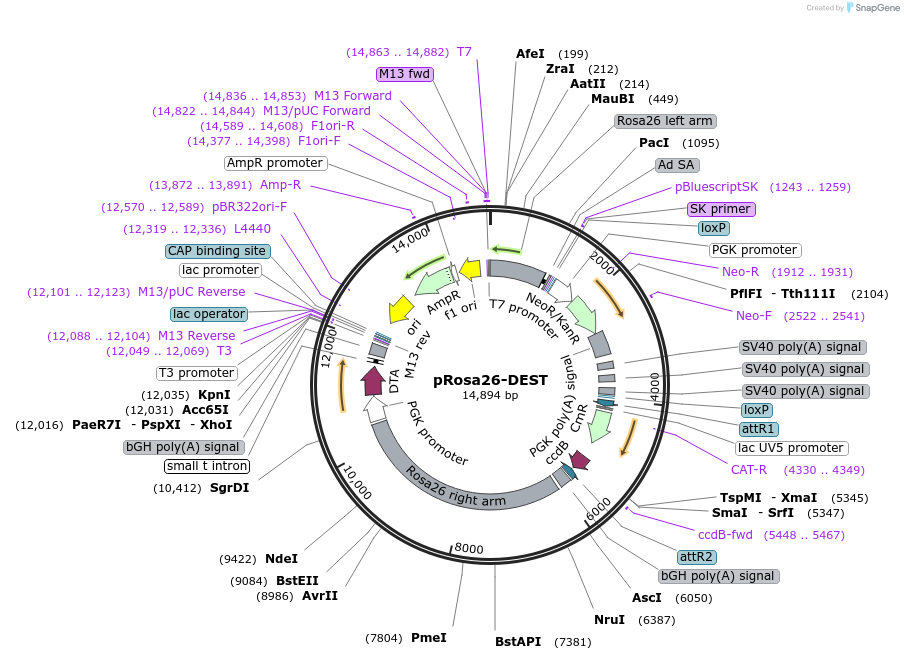
Vector backbonepRosa26-PA
- Backbone size w/o insert (bp) 14820
-
Vector typeMammalian Expression
-
Selectable markersNeomycin (select with G418)
Growth in Bacteria
-
Bacterial Resistance(s)Chloramphenicol and Ampicillin, 25 & 100 μg/mL
-
Growth Temperature30°C
-
Growth Strain(s)ccdB Survival
-
Growth instructionsccdB cells, 30 degrees
-
Copy numberLow Copy
Gene/Insert
-
Gene/Insert nameGateWay conversion cassette
Cloning Information
- Cloning method Gateway Cloning
- 5′ cloning site attR (not destroyed)
- 3′ cloning site attR (not destroyed)
- 5′ sequencing primer n/a
- (Common Sequencing Primers)
Resource Information
-
A portion of this plasmid was derived from a plasmid made bypBigT is from Dr. Costantini of Columbia University. pBigT is based on pRosa26, which is from Dr. Soriano and FHCRC.
-
Articles Citing this Plasmid
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
Depositor Comments
pRosa26-DEST was made by inserting a GateWay conversion cassette into the XhoI site of pBigT, followed by transfer of PacI / AscI fragment of the resulting vector to the pRosa26-PA vector (see http://www.biomedcentral.com/1471-213X/1/4
for details on these vectors). The resulting vector resembles the construct that was used for the R26R Cre reporter mouse made by Phil Soriano in which the expression of lacZ is coupled to the endogenous Rosa26 locus via a splice acceptor site, but only after removal of the lox-STOP-lox cassette (which is actually the neo-R cassette used during the targeting). The only thing changed from the pBigT vectors and the R26R reporter is the cloning strategy, so the resulting knock-in should be as good as these systems, and as good (or as bad) as the use of the Rosa26 locus in any situation.
Stbl3 cells are advisable to use after users have done the LR recombination reaction.
See http://www.hgu.mrc.ac.uk/people/n.hastie_rosa.html
for more information and protocols for this plasmid.
Further advice from the depositing lab:
"Grow single colonies in Amp + Cam at 30 C, definitively not 37. Including the Cam should ensure that spontaneous recombinants that loose the Gateway cassette don't survive...
- Do minipreps and test with the double digest PacI / AscI. This should give 4.9 kb + 9.8 kb. If this is okay, the vector should be okay"
Please note that there are discrepancies between Addgene's quality control sequence and the theoretical sequence supplied by the depositing lab. These discrepancies should not affect the function of the construct.
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
pRosa26-DEST was a gift from Nick Hastie & Peter Hohenstein (Addgene plasmid # 21189 ; http://n2t.net/addgene:21189 ; RRID:Addgene_21189) -
For your References section:
High-efficiency Rosa26 knock-in vector construction for Cre-regulated overexpression and RNAi. Hohenstein P, Slight J, Ozdemir DD, Burn SF, Berry R, Hastie ND. Pathogenetics. 2008 . 1(1):3. 10.1186/1755-8417-1-3 PubMed 19014667