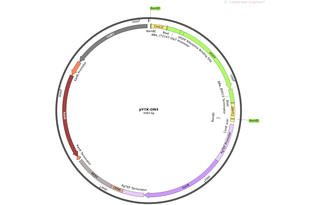
pYTK-DN4
(Plasmid
#180285)
-
PurposeKanR multi-gene yeast expression vector (GFP-dropout)
-
Depositing Lab
-
Sequence Information
Ordering
| Item | Catalog # | Description | Quantity | Price (USD) | |
|---|---|---|---|---|---|
| Plasmid | 180285 | Standard format: Plasmid sent in bacteria as agar stab | 1 | $85 | |
Backbone
-
Vector backbonepYTK084
- Backbone size w/o insert (bp) 1779
- Total vector size (bp) 5083
-
Vector typeBacterial Expression, Yeast Expression
-
Selectable markersgeneticin
Growth in Bacteria
-
Bacterial Resistance(s)Kanamycin, 50 μg/mL
-
Growth Temperature37°C
-
Growth Strain(s)DH5alpha
-
Copy numberHigh Copy
Gene/Insert
-
Gene/Insert nameConLS' - GFP - ConRE' - KanR - CEN6/ARS4
-
SpeciesSynthetic
-
Insert Size (bp)3304
Cloning Information
- Cloning method Restriction Enzyme
- 5′ cloning site BsmBI (destroyed during cloning)
- 3′ cloning site BsmBI (destroyed during cloning)
- 5′ sequencing primer gaattcgcatctagactgatg
- 3′ sequencing primer gacggatcgcttgcctgtaac (Common Sequencing Primers)
Resource Information
-
Supplemental Documents
-
A portion of this plasmid was derived from a plasmid made byA Highly-characterized Yeast Toolkit for Modular, Multi-part Assembly. Lee ME, DeLoache, WC A, Cervantes B, Dueber, JE. ACS Synthetic Biology 2015 DOI: 10.1021/sb500366v
Terms and Licenses
-
Academic/Nonprofit Terms
-
Industry Terms
- Not Available to Industry
Trademarks:
- Zeocin® is an InvivoGen trademark.
Depositor Comments
This plasmid was obtained via Golden Gate assembly of plasmids: pYTK008 + pYTK047 + pYTK073 + pYTK077 + pYTK081 + pYTK084
These plasmids were created by your colleagues. Please acknowledge the Principal Investigator, cite the article in which the plasmids were described, and include Addgene in the Materials and Methods of your future publications.
-
For your Materials & Methods section:
pYTK-DN4 was a gift from Andreas Milias-Argeitis (Addgene plasmid # 180285 ; http://n2t.net/addgene:180285 ; RRID:Addgene_180285) -
For your References section:
A user-friendly and streamlined protocol for CRISPR/Cas9 genome editing in budding yeast. Novarina D, Koutsoumpa A, Milias-Argeitis A. STAR Protoc. 2022 Jun 10;3(2):101358. doi: 10.1016/j.xpro.2022.101358. eCollection 2022 Jun 17. 10.1016/j.xpro.2022.101358 PubMed 35712010